ITEM SPECIFICS
-
Brand
Rhinodelight
-
origin
Republic of Korea
-
Size(Capacity)
18ml
-
Weight
30g
-
Features
cleans foreign substances in the nose without preservatives, antibiotics, or steroids.
-
Condition
Liquid
-
Gender
none
-
Material
Saline , Methyleneblue
-
Color
None
-
Function
Removal of foreign substances
PRODUCT DESCRIPTION
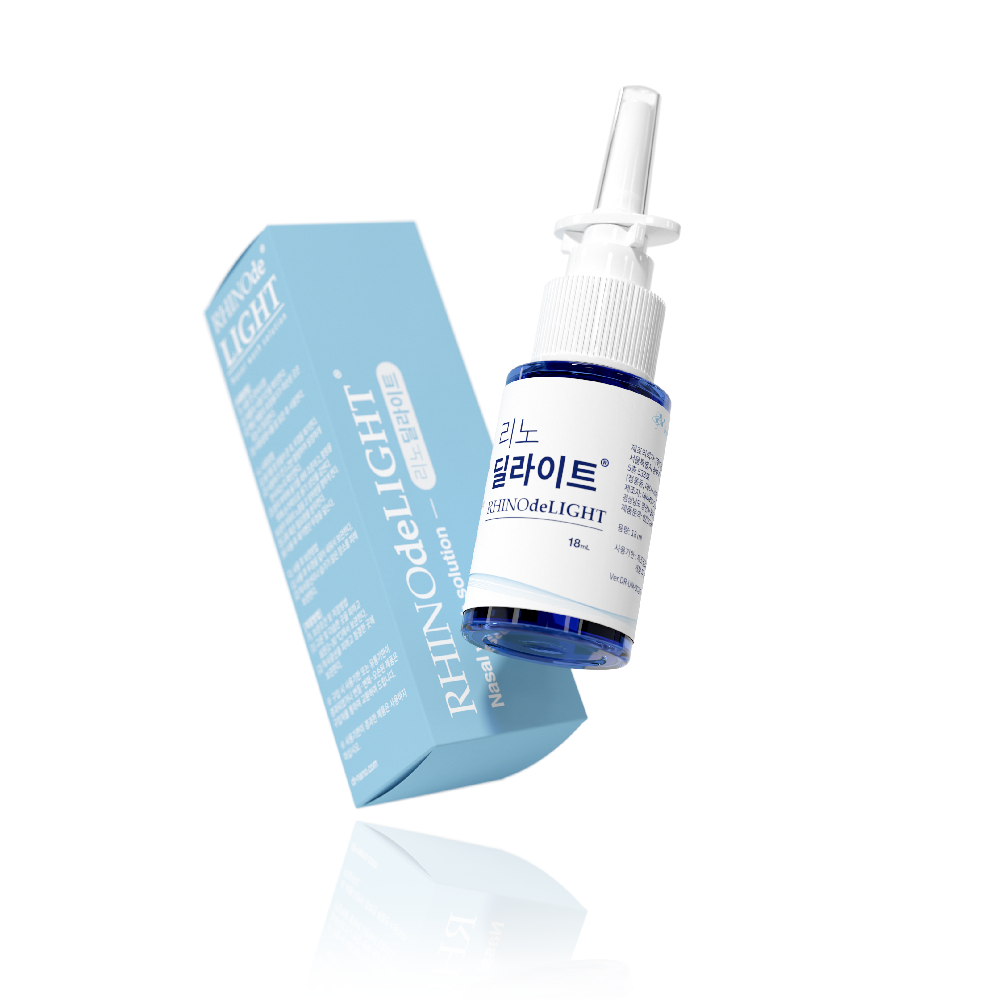
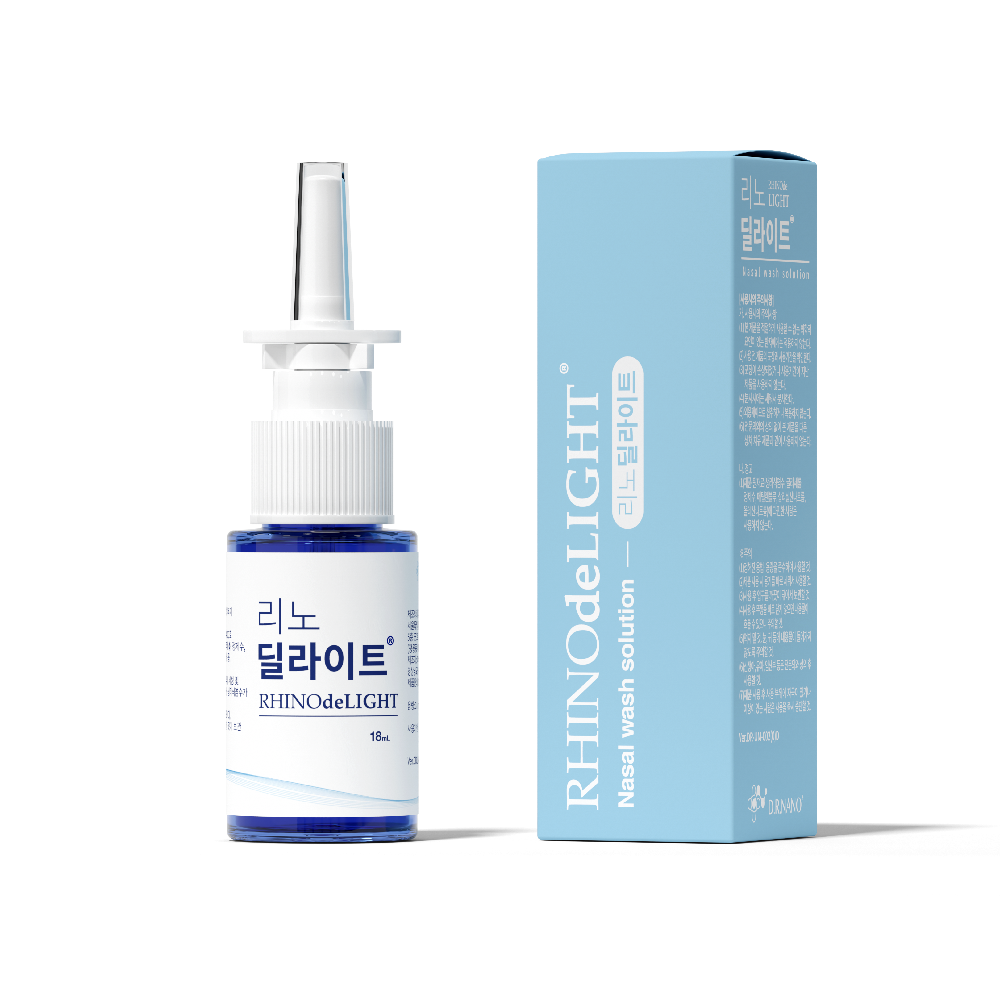
DRNANO Rhinodelight
BRAND: Rhinodelight
Manufacturer's name: DRNANO
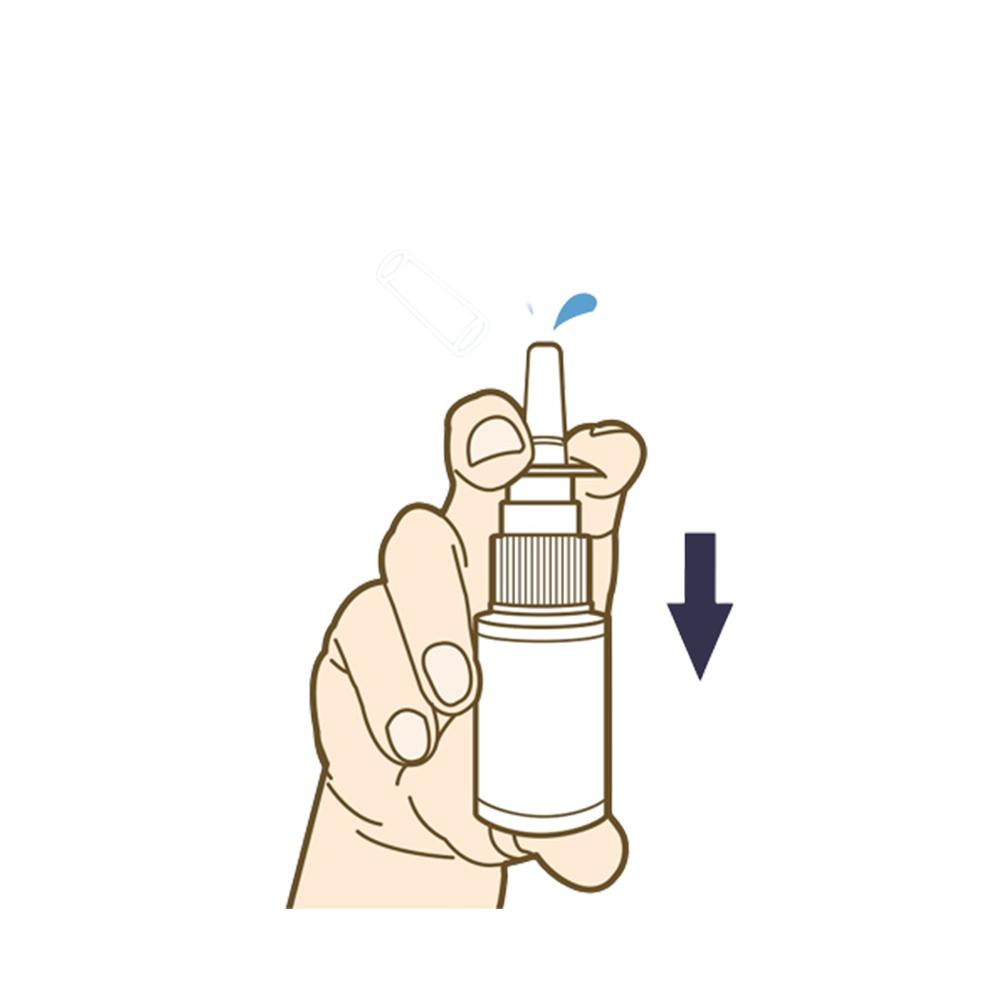
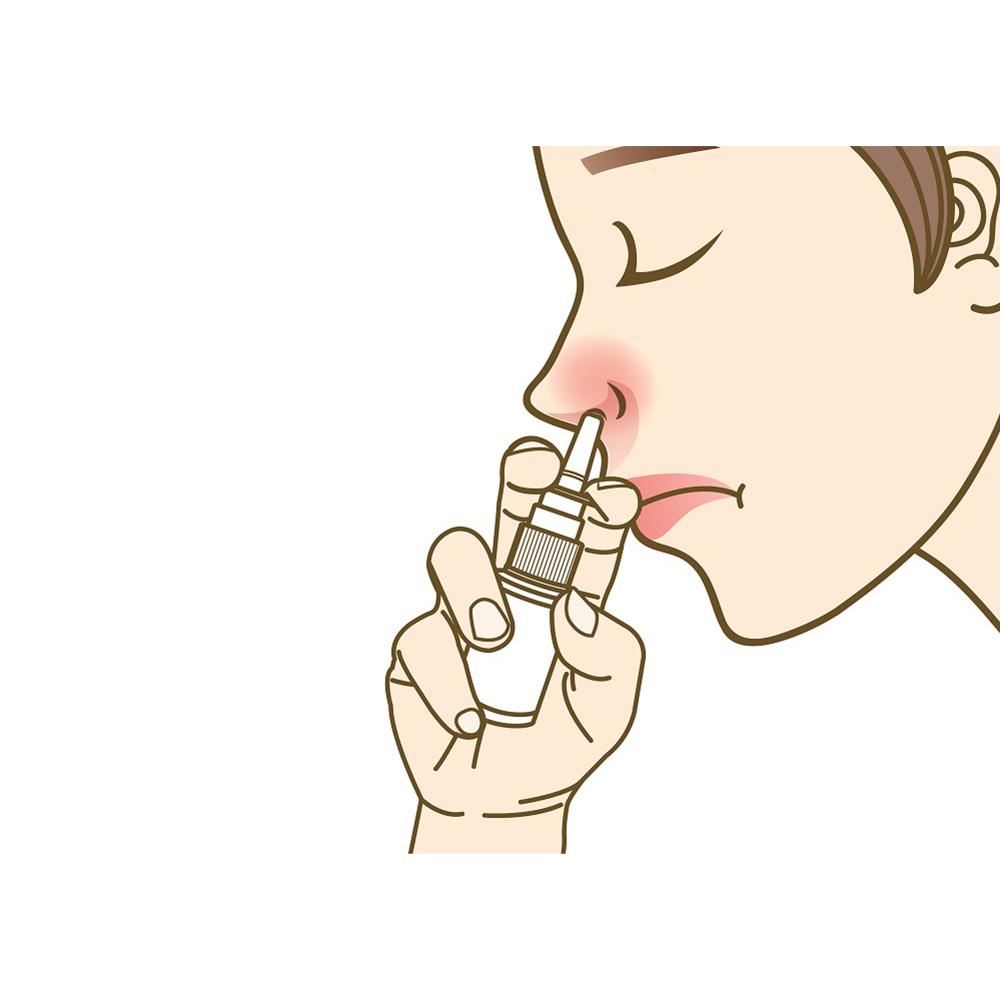
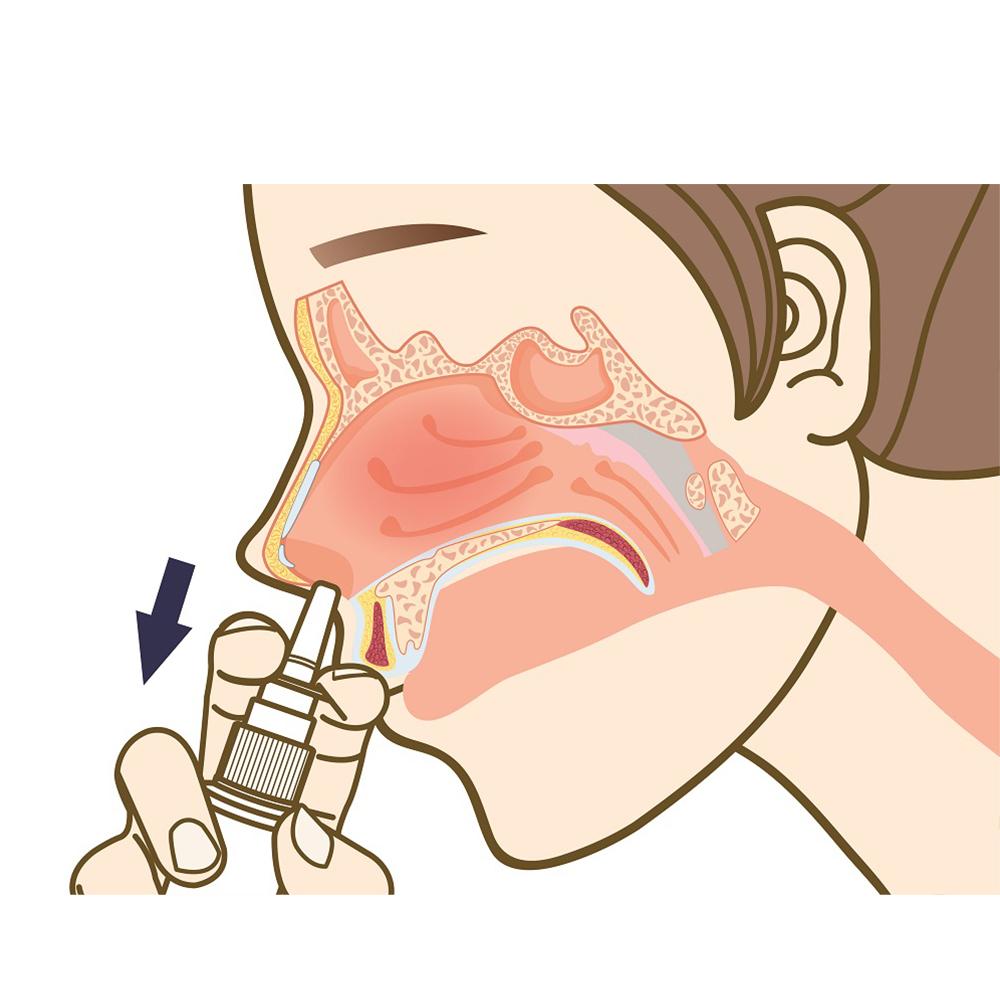
This product is a saline wound dressing material containing methylene blue that is used to clean damaged nasal mucosa and remove foreign substances. It moisturizes the nasal mucosa in the nose and protects the skin.
Methylene blue has been studied as an anti-oxidant, anti-microbial, and photodynamic effect as various therapeutic substances. Currently, methylene blue 0.5% (5,000 ppm) injection is used as a treatment for methemoglobinemia.
In the case of this product, phosphate buffered saline, purified water, glycerol, and methylene blue complex aqueous solution (based on methylene blue content of 4.6 ppm) are the main ingredients, and it is easy to clean the nasal mucosa and remove foreign substances by using a polyethylene container that can be sprayed into the nose.
Methylene blue has been studied as an anti-oxidant, anti-microbial, and photodynamic effect as various therapeutic substances. Currently, methylene blue 0.5% (5,000 ppm) injection is used as a treatment for methemoglobinemia.
In the case of this product, phosphate buffered saline, purified water, glycerol, and methylene blue complex aqueous solution (based on methylene blue content of 4.6 ppm) are the main ingredients, and it is easy to clean the nasal mucosa and remove foreign substances by using a polyethylene container that can be sprayed into the nose.
[Suggested Use] Use to 1~3 times per day
[Specifications]
∙ Microbiological Limit Test: > 99,99 % reduction
∙ Phosphate buffered saline (pH 7.4)
∙ Additives (Methylene blue/sodium salicylate/sodium oleate mixture)
∙ Methylene blue content: *4.6ppm ±35%
∙ Volume : 18mL
∙ Microbiological Limit Test: > 99,99 % reduction
∙ Phosphate buffered saline (pH 7.4)
∙ Additives (Methylene blue/sodium salicylate/sodium oleate mixture)
∙ Methylene blue content: *4.6ppm ±35%
∙ Volume : 18mL
R&D CERTIFICATE
-
- medical devices quality management system
- institute of grobal certification
- 22.01.28
- 인증서보기
PAYMENTS DETAILS
This supplier supports payments for offline orders
- OT
- Telegraphic Transfer : T/T
- Name : Tae Yeong Lim
SHIPPING
Shipping from :
Republic of Korea
- 161 Jeongneung-ro, Seongbuk-gu, Seoul (02708)
D.R.NANO Co., Ltd.
The person in charge
Jay Junkeun ChangAddress
161 Jeongneung-ro, Seongbuk-gu, Seoul (02708)
QR CODE
D.R.NANO Co., Ltd.
Introduction
Our company is a second-generation startup model company established as a joint venture between a research institute, a company and a researcher by investing in kind the original technology of the Korea Institute of Science and Technology (KIST).
We specialize in R&D of high-tech products that extend life and improve quality of life for chronic diseases such as skin and ophthalmic diseases and cancer by applying drug/excipient nano-repositioning platform technology.
We are currently preparing for an IPO based on clinical trials and research results.
2022
01.ISO 13485 accreditation
02.Selected customized support program related to global business establishment by KOTRA.
03.Certified of Medical device Manufacturing for RHINOdeLIGHT
2021
07.Certified of medical device Manufacturing for RHINOdeLIGHT/RHINOdeLIGHT Combo(for export)
12.Head office relocation (Songpa -> Seongbuk)
Company affiliated research center relocation (Sonpa -> Jungnang)
2020
01.Head office relocation (Seongbuk -> Songpa)
03.Certified as a small and medium Business
03.Certified of Good Manufacture Practices (GMP)
04.Certified of Medical device Manufacturing for DERMAdeLIGHT
09.Certified as a LMO
2019
01.Registered as a new technology start-up company
01.Reselected as a start-up business support program by Seoul Business Growth Center
02.Certified as a venture company
04.Medical device clinical trial approval
05.Started medical device clinical trial
2018
02.Selected as a start-up business support program by Seoul Business Growth Center
04.Head office relocation (Yongsan -> Seongbuk)
05.Selected as an investment-linked R&D program by commercializations Promotion Agency for R&D Outcomes
06.Company affiliated research center certified
08.Certified of Good Manufacture Practices (Clinical trial GMP)
2017
11.Good Manufacturing Practices (GMP) Production process development
2016
11.Acne treatment development research
2015
12.Established D.R.NANO Co., Ltd
-
- Business Type :
- Manufacturer
-
- Main Product :
- Nasal spray, Wound Dressing
-
- Established :
- 2015-12-22
-
- Total Annual Revenue :
- Less than 100 million (KRW)
-
- Total Employees :
- Less than 5
R&D CERTIFICATE
-
- medical devices quality management system
- institute of grobal certification
- 22.01.28
- 인증서보기
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- Less than 100 million (KRW)
-
- Total export revenue (previous year in USD)
- 0
-
- Number of foreign trade employees
- Less than 5
COMPARISON TO SIMILAR ITEMS more
- No Items