ITEM SPECIFICS
-
Brand
SURE&EARLY
-
origin
Republic of Korea
-
Size(Capacity)
43.8g
PRODUCT DESCRIPTION
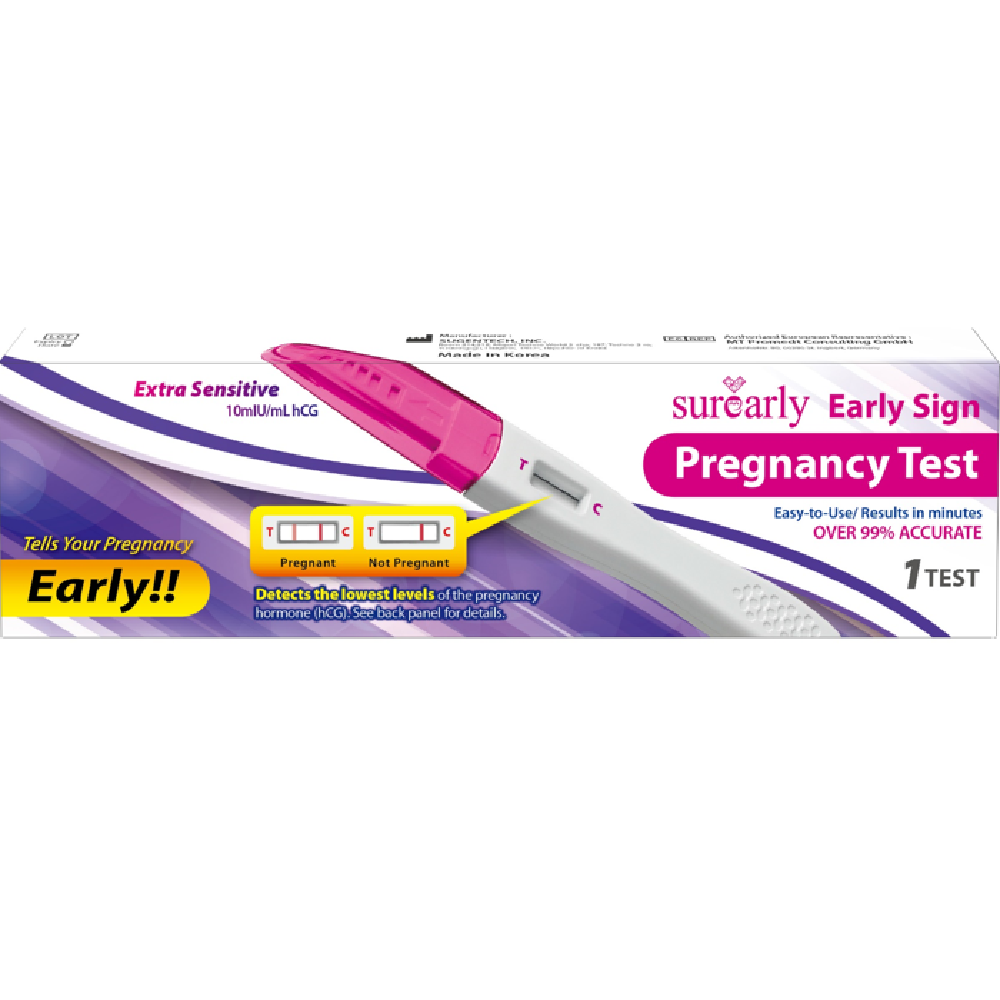
It is an in vitro diagnostic medical device that uses hCG hormone in the urine as an antigen-antibody immunochromatographic method to check pregnancy.
This product is an in vitro diagnostic medical device that detects pregnancy by detecting hCG hormone in urine. It is judged as positive if the lowest hCG concentration with positive detection rate is more than 10mlu / ml.
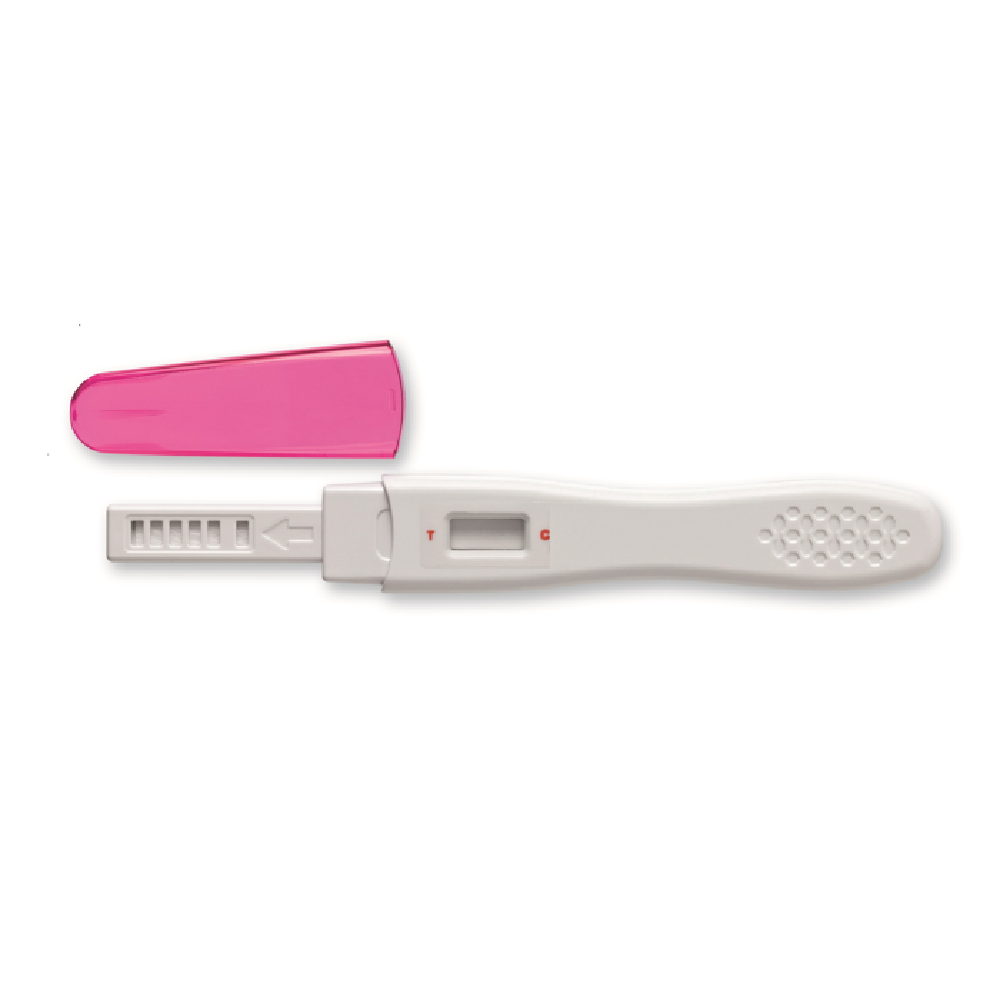
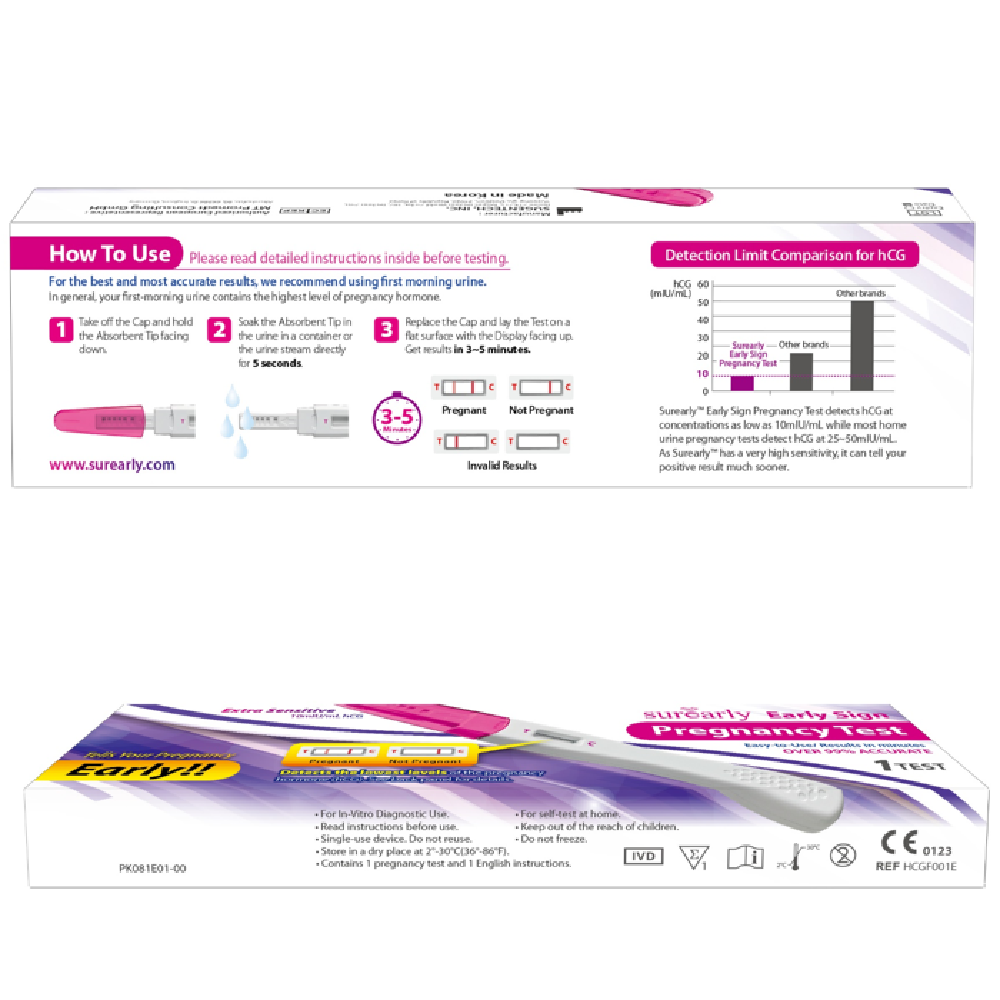
How to use
1. Sample preparation
- Use urine as a specimen and recommend using the first urine as soon as possible.
- Urine can be taken in a clean container or used directly in flowing urine.
2. Preparation before inspection
- Remove the aluminum foil package to remove the tester.
- Open the lid of the tester.
3. Inspection Process
- Hold the tester handle and point the absorption bar down.
- Wet the absorbing rod for 5 seconds to the urine collected in flowing urine or in a clean container.
4. Results determination and quality control
1. Sample preparation
- Use urine as a specimen and recommend using the first urine as soon as possible.
- Urine can be taken in a clean container or used directly in flowing urine.
2. Preparation before inspection
- Remove the aluminum foil package to remove the tester.
- Open the lid of the tester.
3. Inspection Process
- Hold the tester handle and point the absorption bar down.
- Wet the absorbing rod for 5 seconds to the urine collected in flowing urine or in a clean container.
4. Results determination and quality control
R&D CERTIFICATE
PAYMENTS DETAILS
This supplier supports payments for offline orders
- Telegraphic Transfer : T/T This payment are available by Gobiz.com system.
- P@yGOS(PayPal) Contact Payment Manager
- Name : Jiyeong Kwak
SHIPPING
Shipping from :
Republic of Korea
- 187, Techno 2-ro,Yuseong-gu, Daejeon, Republic of Korea (34025)
Sugentech
The person in charge
Mijin SonAddress
187, Techno 2-ro,Yuseong-gu, Daejeon, Republic of Korea (34025)
Introduction
SUZENTECH Co., Ltd. is a 28th institute of science and technology information and communication department established in 2011 by transferring technology of ETRI's "Ubiquitous Biochip Reader" technology.
Since its establishment, we have accumulated bio-and nano-based technologies and commercialized a variety of in vitro diagnostic systems to become a comprehensive in vitro diagnostic company based on fusion technology including bio, nano and IT.
Based on its internalized bio-nano-IT fusion technology and commercialization capability, SuzenTec is opening up a personalized diagnosis period through the development of automated diagnostic equipment.
It is a leader in smart healthcare systems that supports the fourth industrial revolution in healthcare.
-
- Business Type :
- Manufacturer
-
- Main Product :
- Surearly Digital Pregnancy Test
-
- Established :
- 2011-12-09
-
- Total Annual Revenue :
-
- Total Employees :
- 11~50 people
R&D CERTIFICATE
COMPANY ENVIRONMENT
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
-
- Total export revenue (previous year in USD)
- 3
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
- MEMBER
- Sugentech Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★