ITEM SPECIFICS
-
Brand
Model No. 14-1225Unbranded
-
origin
Republic of Korea
-
Size(Capacity)
1box 96tests
PRODUCT DESCRIPTION
Description
Reagent used by in vitro diagnosis analyzer, which tests for
M. tuberculosis complex in human expectoration, urine,
tissue and blood and culture samples utilizing real-time
polymerase chain reaction (PCR) tests
Components
Clinical trial
Reagent used by in vitro diagnosis analyzer, which tests for
M. tuberculosis complex in human expectoration, urine,
tissue and blood and culture samples utilizing real-time
polymerase chain reaction (PCR) tests
Components
Clinical trial
- The above test was performed in the Asan Clinical Trial Center.
There were total 400 clinical samples (positive 200, negative 200)
and the trial showed 96% agreement with the Gold Standard
for Tuberculosis diagnosis, and 100 % agreement for the negative.
(This data is reported to the Korean FDA in the trial report submitted
for the product approval)
Excellent Reproducibility
- If there seems to be a difference between the result of the molecular
diagnosis and the definite diagnosis, try EuDx-MTB detecion kit. - We guarantee for high sensitivity, specificity, and reproducibility.
Fast Diagnosis
- In the diagnosis of the M. tuberculosis, the culture test which
is the Gold Standard takes about 10 days to 8 weeks. - However, EuDx-MTB Detection Kit which uses the real-time
PCR gets the result within 2 hours. - Fast diagnosis can reduce the treatment time.
Features
1) High performance (Responsiveness&Specificity): Fusion of responsive PCR technology (Real-time PCR + Nested PCR)
: Tests for 2 types of genes (Multiplex PCR)
: Uses high efficiency enzymes
2) Pollution prevention : Performs PCR twice in one tube
: Applies UDG (Uracil-DNA glycosylase) system
3) Highly stable: Reagent remains stable more than a year after production
: Tests for 2 types of genes (Multiplex PCR)
: Uses high efficiency enzymes
2) Pollution prevention : Performs PCR twice in one tube
: Applies UDG (Uracil-DNA glycosylase) system
3) Highly stable: Reagent remains stable more than a year after production
Our technology is related to the manufacturing of the reagent used in molecular analysis of tuberculosis, and it is a molecular diagnostic technology that tests for Mycobacterium Tuberculosis (MTB) by utilizing Real-time PCR. This technology possesses relatively high responsiveness and specificity compared to other existing products.
Since even a small amount of MTB is analyzed after going through primary PCR, this product is advantageous in the initial detection of MTB infections and detects even miniscule amounts of MTB. Also, the application of Dual target technology decreased false positive and false negative results significantly. There was no notable statistical difference between the results of Roche COBAS, product with the highest market share both domestically and internationally, and our clinical trial results, which is evidence that there is no difference in quality between our product and the number one product in the world.
More than 10% cheaper compared to other products both domestically and internationally, and extremely competitive due to being more than 100 times more responsive compared to other products in internal test results.
More than 10% cheaper compared to other products both domestically and internationally, and extremely competitive due to being more than 100 times more responsive compared to other products in internal test results.
Specification
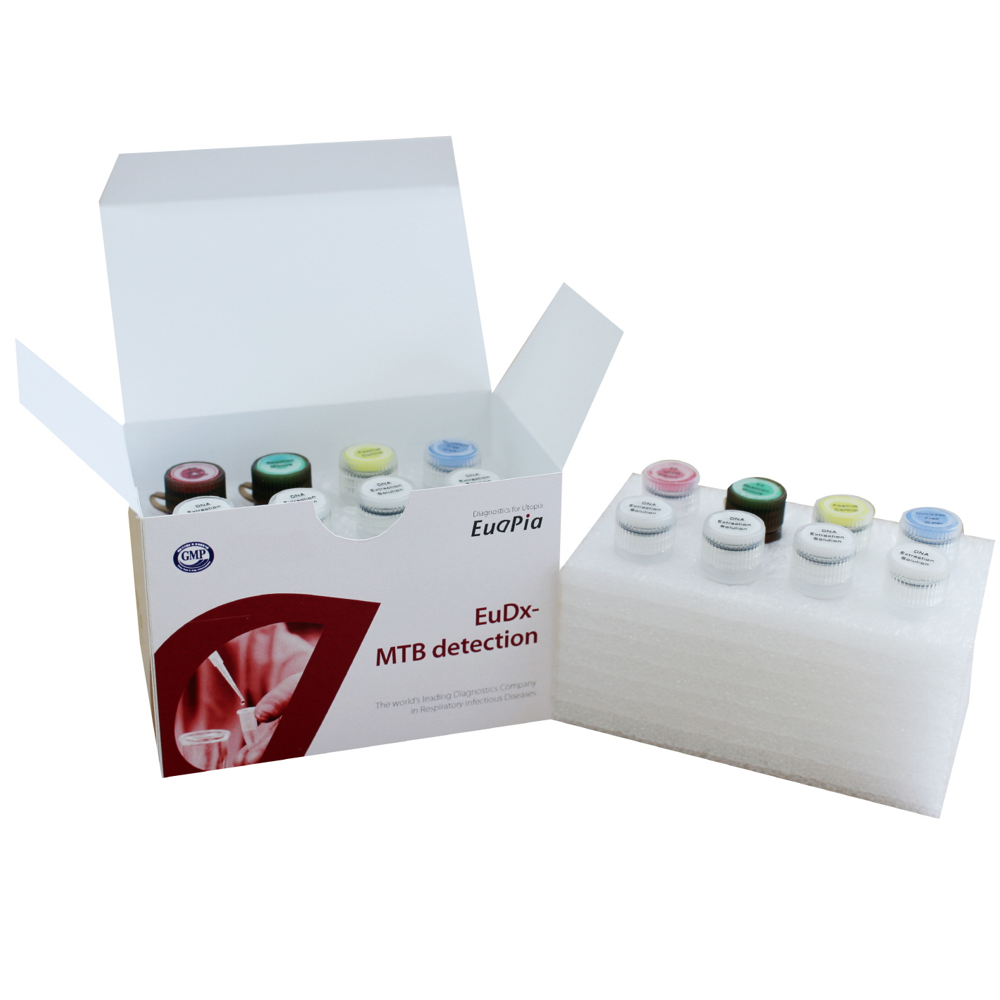
Product Composition
- 2X qPCR Premix
- 4X Reaction Mixture(Primer/Probe + Internal Control)
- Positive Control DNA
- Nuclease Free Water
- DNA Extraction Solution
- 2X qPCR Premix
- 4X Reaction Mixture(Primer/Probe + Internal Control)
- Positive Control DNA
- Nuclease Free Water
- DNA Extraction Solution
Others
Product Specification
-1box 96tests
-1box 96tests
PAYMENTS DETAILS
This supplier supports payments for offline orders
- Letter of Credit : L/C(sight)
- Telegraphic Transfer : T/T
- Name : In Soo Kim
SHIPPING
Shipping from :
Republic of Korea
- 194-41 Osongsaengmyeong 1-ro Osong-eup Heungdeok-gu, Cheongju-si, Chungcheongbuk-do Korea (28160)
EuDiPia Co. Ltd.
The person in charge
In Soo KimAddress
194-41 Osongsaengmyeong 1-ro Osong-eup Heungdeok-gu, Cheongju-si, Chungcheongbuk-do Korea (28160)
Introduction
Eudipia Co., Ltd. is a professional diagnostics company mainly providing molecular diagnosis services. We started with the diagnosis of tuberculosis and we plan to expand our diagnostic products to various fields.
Eudipia Co., Ltd. was established with the goal of becoming the absolute best
in the world in the diagnosis of tuberculosis.
Since then we plan to expand our diagnostics to the fields of respiratory diseases
and early cancer diagnosis.
We named our company Eudipia in the hope to create an utopia through diagnosis.
Furthermore, Swift and precise Fully Automated POCT system development for improved
user convenience is currently in development, and diagnostics for respiratory viruses,
MDR TB, venereal diseases, dengue, malaria, etc. are set to be released in 2017.
Next, Eupidia Co., Ltd. plans to branch out into the world by establishing contracts with dealers and distributors through discovery of transaction lines country by country.
-
- Business Type :
- Manufacturer
-
- Main Product :
- EuDx™-MTB Detection Kit, MTB One-tube Nested Multiplex real-time PCR
-
- Established :
- 2012-09-01
-
- Total Annual Revenue :
- 6 million to 10 billion (KRW)
-
- Total Employees :
- 11~50 people
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- 6 million to 10 billion (KRW)
-
- Total export revenue (previous year in USD)
- 10
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
-
 GOLD
GOLD
- EuDiPia Co. Ltd. Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★




![EuDx™-ufPCR System[ultra fast system]](/image/goodsImage.do?goods_no=GS2017110617520&image_se_code=MAIN_THUMB174)