ITEM SPECIFICS
-
Brand
Model Submerged SystemSubmerged System
-
origin
Republic of Korea
-
Size(Capacity)
Stated in catalogue
-
Material
Titanium
-
Function
Tooth root replacement
PRODUCT DESCRIPTION
[Product Description]
· The CSM Submerged Implant System includes various one-stage Fixtures and two-stage Fixtures made of titanium.
· These implants are surgically inserted into the upper and/or lower jawbone and serve as a tooth root replacement providing a stable foundation for restorations.
[Product Precautions]
· Surgical technique for endosseous dental fixture implant requires special and complex procedures.
· Formal training for fixture placement is recommended.
· Important : Determine local anatomy and suitability of the available bone for fixture placement.
· Thorough screening of prospective fixture must be performed.
· Panoramic and periapical radiograph for visual inspection are essential to determine anatomical landmarks, occlusal condition, periodontal
status and adequacy of bone.
status and adequacy of bone.
· Lateral cephalometric radiographs, computerized axial tomograghy and tomogram could be instructive.
· Appropriate radiography, direct palpation and visual inspection of the fixture site are necessary for planning or treatment prior to use fixtures.
[Directions for use]
· The surgical procedure should be done under aseptic condition with specially designed sterile surgical instruments.
· The sterilized implants have to be delivered precisely from package to preparation site.
· An electrical surgical drilling system with internal or external irrigation is recommended.
· An electrical surgical drilling system with internal or external irrigation is recommended.
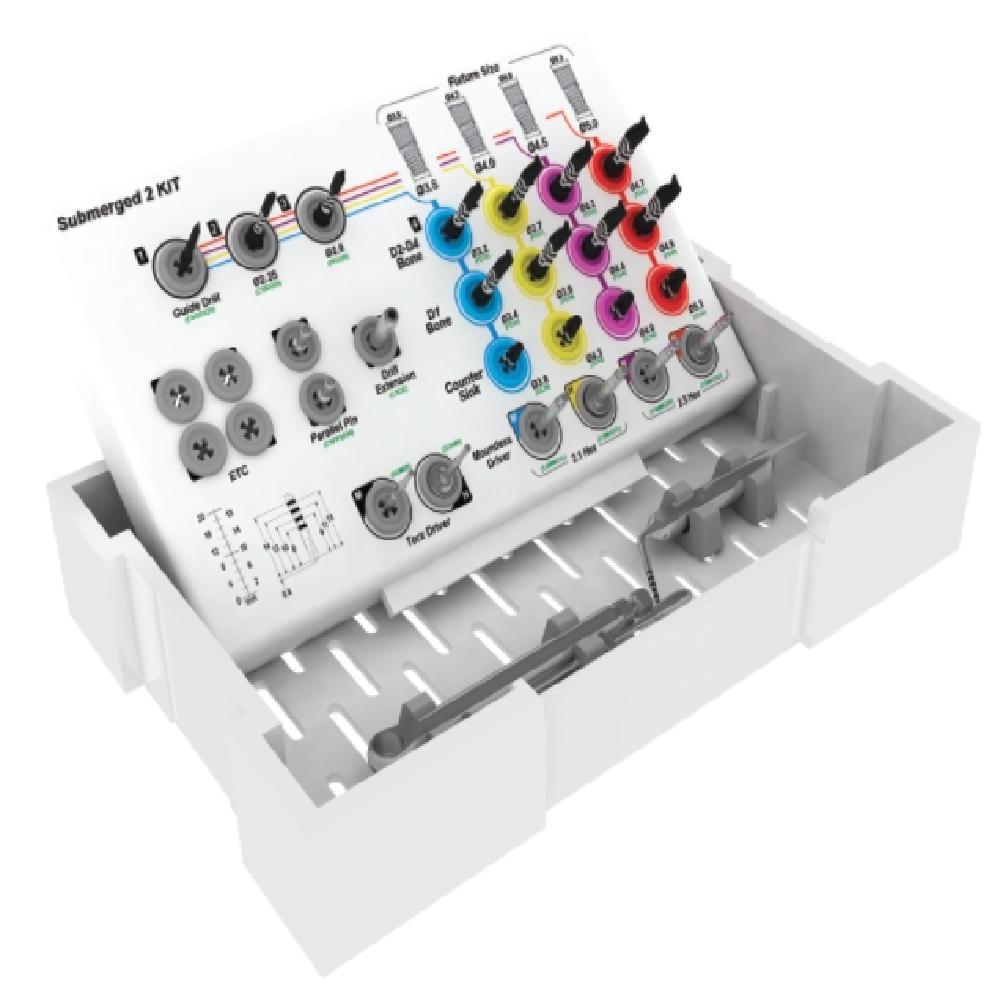
· Prescribed drilling sequences (Guide drill – Initial drill – Pre-final drill - Final drill – Reamer) or combination of surgical tools.
· In case of Hard Bone(D1) please use CSM D1 Drill or the drill of next diameter.
· 70Ncm torque power is recommended and Parallel Pin is used for measuring the direction and the depth of the hole.
· 70Ncm torque power is recommended and Parallel Pin is used for measuring the direction and the depth of the hole.
· Fixture in the ampul should be placed up by using Mountless Driver and be planted into the bone.
· Handpiece or Ratchet Wrench is usable to plant.
· Insertion depth of CSM fixture shall be 0.2mm below than the bone level.
· When the fixture is fully seated, carefully remove Mountless Driver and place Cover Screw or Healing Abutment on the fixture.
· Then close tissue flap and suture it.
· The healing period will be 45~90days for lower jaw, 90~180days for upper jaw.
(Please be careful not to lose balance in drilling work during operation)
(Please be careful not to lose balance in drilling work during operation)
[Product Specification]
· CSM dental implant is used to restore lost chewing ability and improve aesthetics by replacing missing tooth roots of edentulous patients.
· Submerged Implant
(Sub1, Sub3: abutment could be comaptible with Osstem-TS, Dentium-Super line, Implantium More than 90% CSM customers are using this system)
(Sub1, Sub3: abutment could be comaptible with Osstem-TS, Dentium-Super line, Implantium More than 90% CSM customers are using this system)
· Surgical kit: All the drills and drivers have duration for 30~50 times.
· Qualification: ISO13485, CE mark, Korea KFDA, USA FDA, CFDA Certificate approval
· Qualification: ISO13485, CE mark, Korea KFDA, USA FDA, CFDA Certificate approval
[Surface treatment]
1. RBM surface: It is most common surface among the implant manufacturers. The surface is treated by HA powder.
- Reference: https://synapse.koreamed.org/DOIx.php?id=10.4047/jkap.2018.56.1.1&vmode=PUBREADER
2. SLA surface: https://www.ncbi.nlm.nih.gov/pubmed/27540839
3. Laser surface:
Comparing with anodized
https://www.ncbi.nlm.nih.gov/pubmed/21778844
Comparing with SLA
https://synapse.koreamed.org/Synapse/Data/PDFData/0170JAP/jap-6-302.pdf
https://www.ncbi.nlm.nih.gov/pubmed/25177474
Comparing with Active SLA
https://synapse.koreamed.org/Synapse/Data/PDFData/0170JAP/jap-8-110.pdf
https://www.ncbi.nlm.nih.gov/pubmed/27093590
Clinical Studying
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5917109/pdf/jap-10-163.pdf
https://www.ncbi.nlm.nih.gov/pubmed/29713438
You can find further details on our web-site www.csmimplant.com
R&D CERTIFICATE
PAYMENTS DETAILS
This supplier supports payments for offline orders
- Telegraphic Transfer : T/T
- Name : Manager
SHIPPING
Shipping from :
Republic of Korea
- 47 Gyeongdae-ro 17-gil, Buk-gu, Daegu (41566)
CSM Implant
The person in charge
Sungam ChoAddress
47 Gyeongdae-ro 17-gil, Buk-gu, Daegu (41566)
QR cord
CSM Implant
Introduction
Thank you for visiting CSM Implant!
Welcome to CSM Implant and thank you for visiting our web site.
Since its foundation in 2000, CSM Implant has provided high quality dental products and service with the clean and safe production environment.
Founder/President Sungam Cho who is a professor of, department of prosthodontics, college of Dentistry, Kyung-pook national University, which is one of the leading national universities in Korea. All products are developed by many of experienced dentists and dental specialists in dental implant industry.
Our energetic and passionate team members keep discovering the innovation in dental implant system. CSM’s top priority is to meet both dentists’ and patients’ satisfaction with the better implant system based on long-term research and development experience.
CSM Implant aims to produce not only superior in quality, but also reasonable in price with user friendly service. All of our uniquely designed dental implants minimize the possible damage on teeth such as loss of surrounding teeth, migration of teeth and teeth structure loss.
We will continuously develop clean and safe implant system with our sincere heart and will consistently pursue our number one object which is to meet user satisfaction in terms of esthetic and functional aspect.
Thank you for taking your interests and time for CSM Implant, we hope to hear from you soon.
-
- Business Type :
- Manufacturer
-
- Main Product :
- Implant product
-
- Established :
- 2000-01-24
-
- Total Annual Revenue :
-
- Total Employees :
- 11~50 people
R&D CERTIFICATE
-
- ISO 13485
- tuv_sud
- 2019.1.8
- 인증서보기
-
- CE Certificate Full Quality Assurance System
- EC Directives
- 2017.03.10
- 인증서보기
-
- Certificate of 510(k)
- FDA
- 2011.3.22
- 인증서보기
-
- CE Certificate Full Quality Assurance System
- EC Directives
- 2017.3.10
- 인증서보기
-
- ISO 13485
- tuv_sud
- 2019.01.08
- 인증서보기
-
- FDA
- office of device evaluation
- 11.12.28
- 인증서보기
-
- Certificate of GMP
- ktl
- 19.04.01
- 인증서보기
-
- CE Certificate
- CE
- 17.03.10
- 인증서보기
-
- FDA
- Office of device evaluation
- 11.12.28
- 인증서보기
-
- Certificate of patent
- Korean Intellectual Property Office
- 08.01.15
- 인증서보기
COMPANY ENVIRONMENT
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
-
- Total export revenue (previous year in USD)
- 3
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
-
 PLATINUM
PLATINUM
- CSM Implant Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★