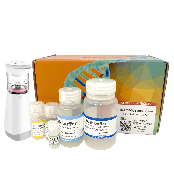
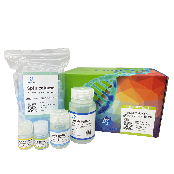
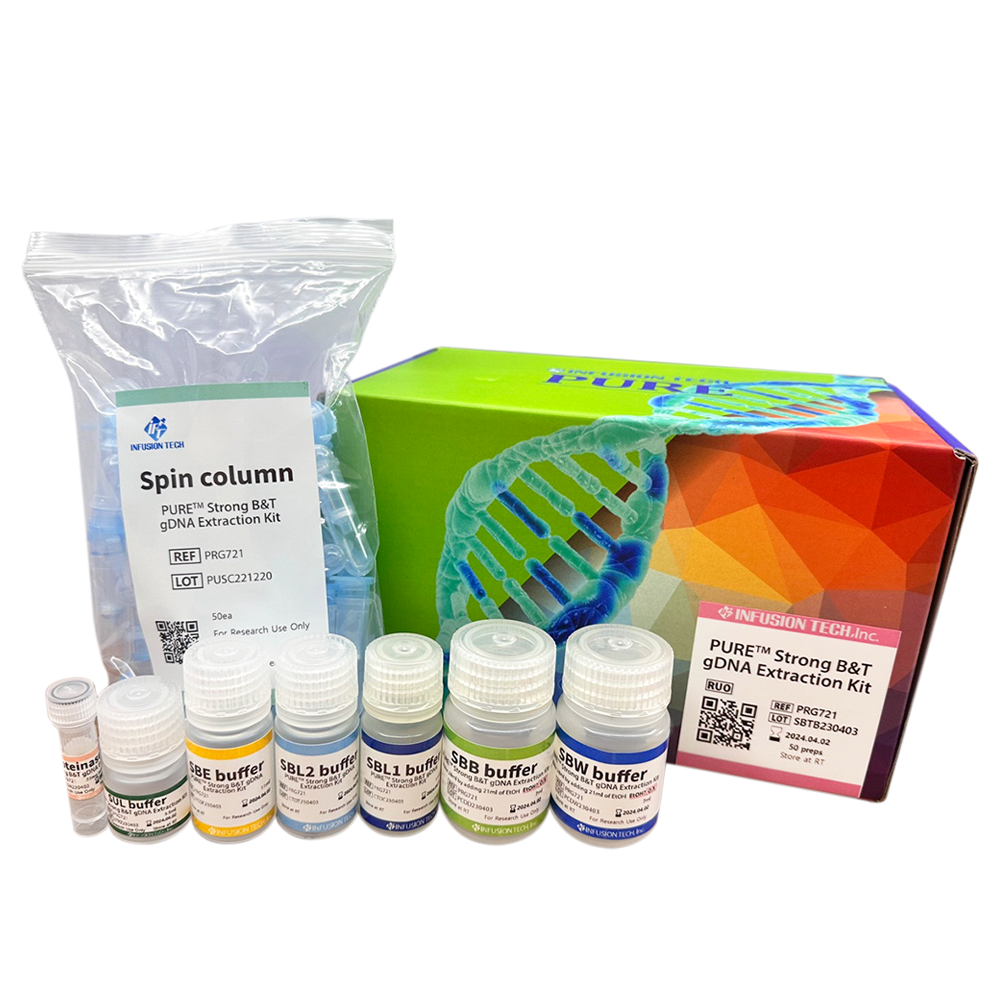
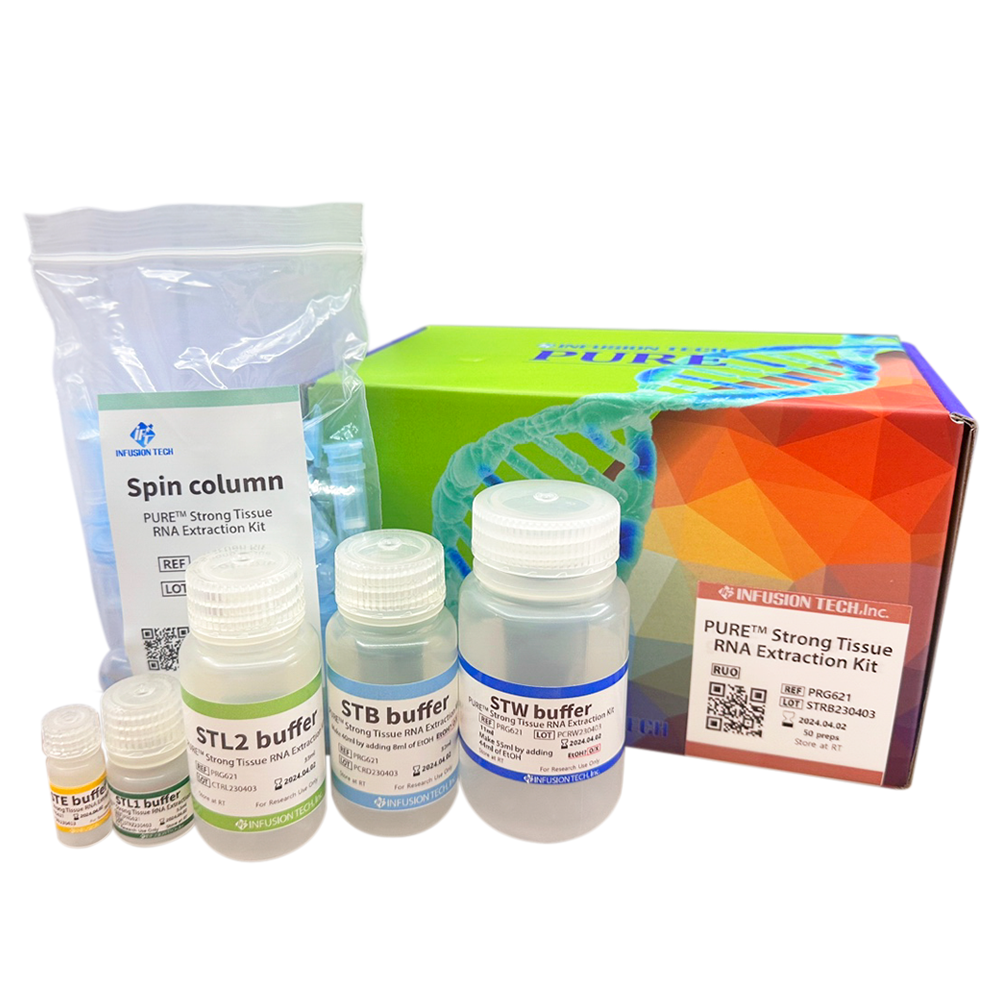
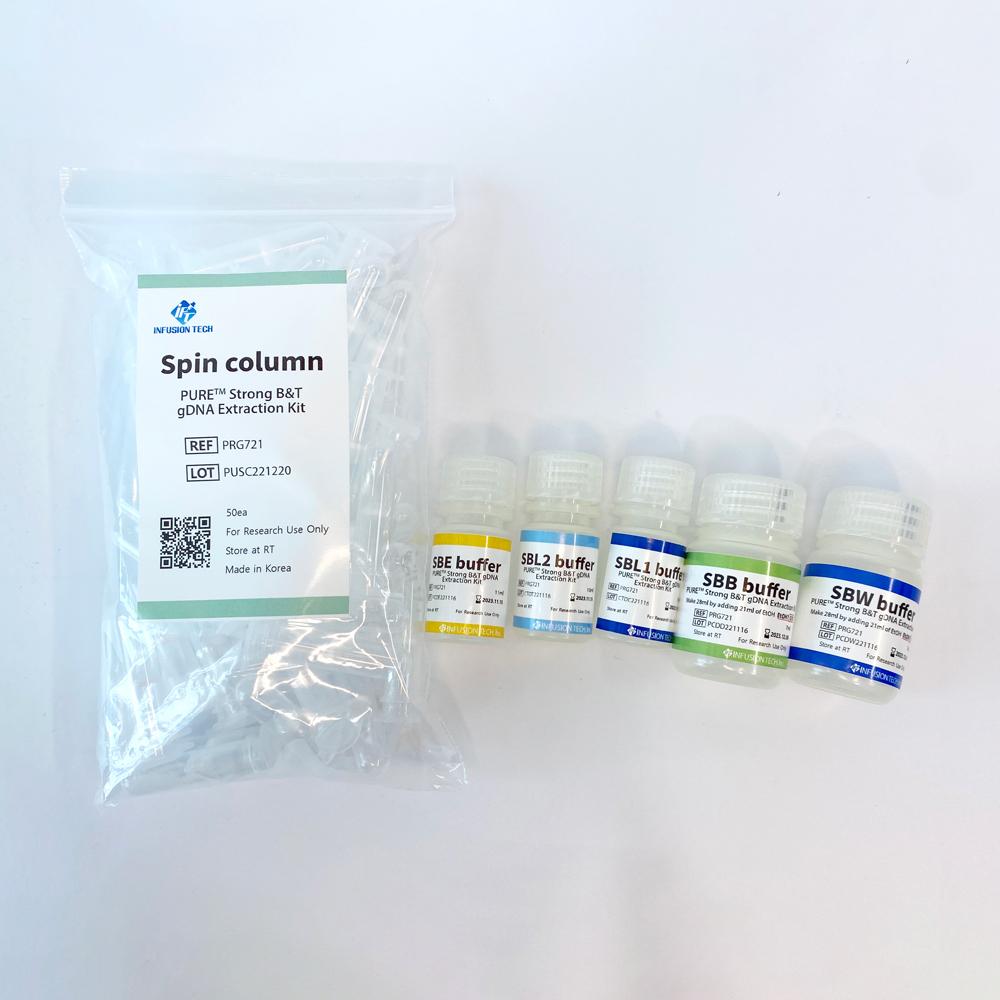
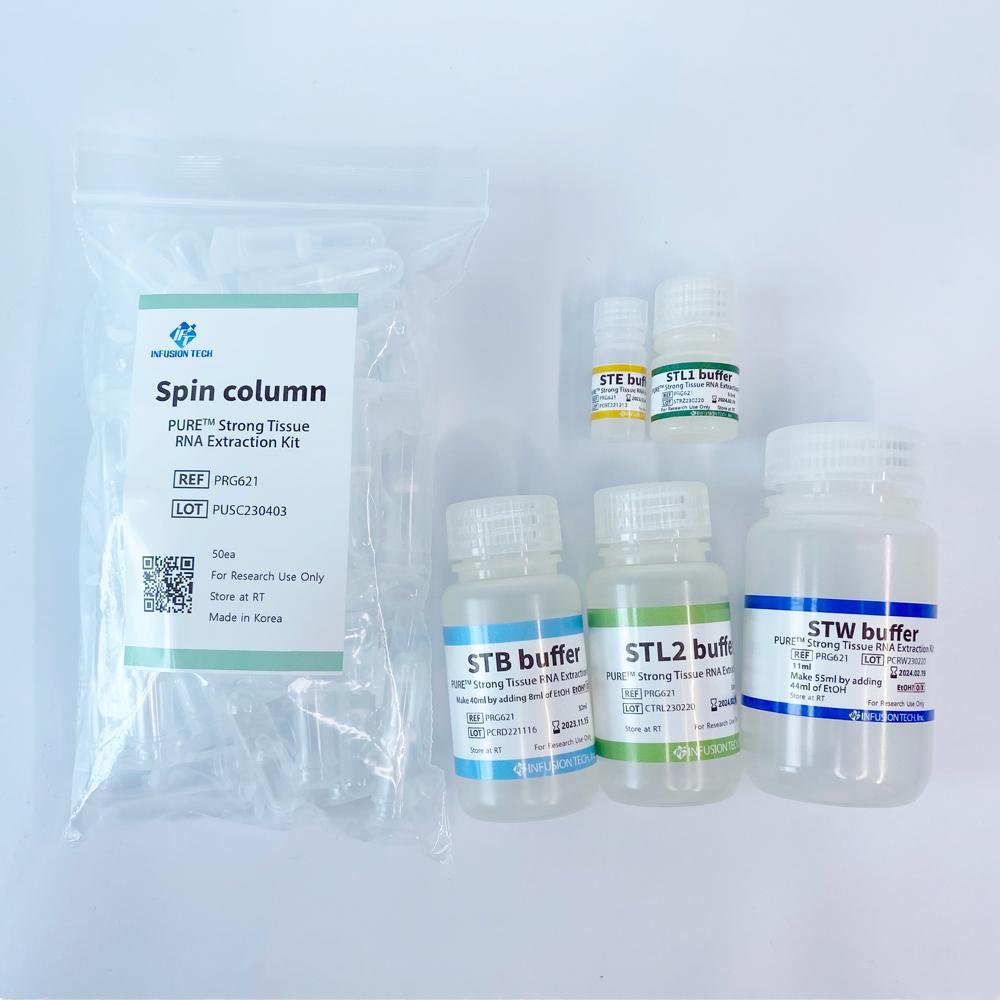
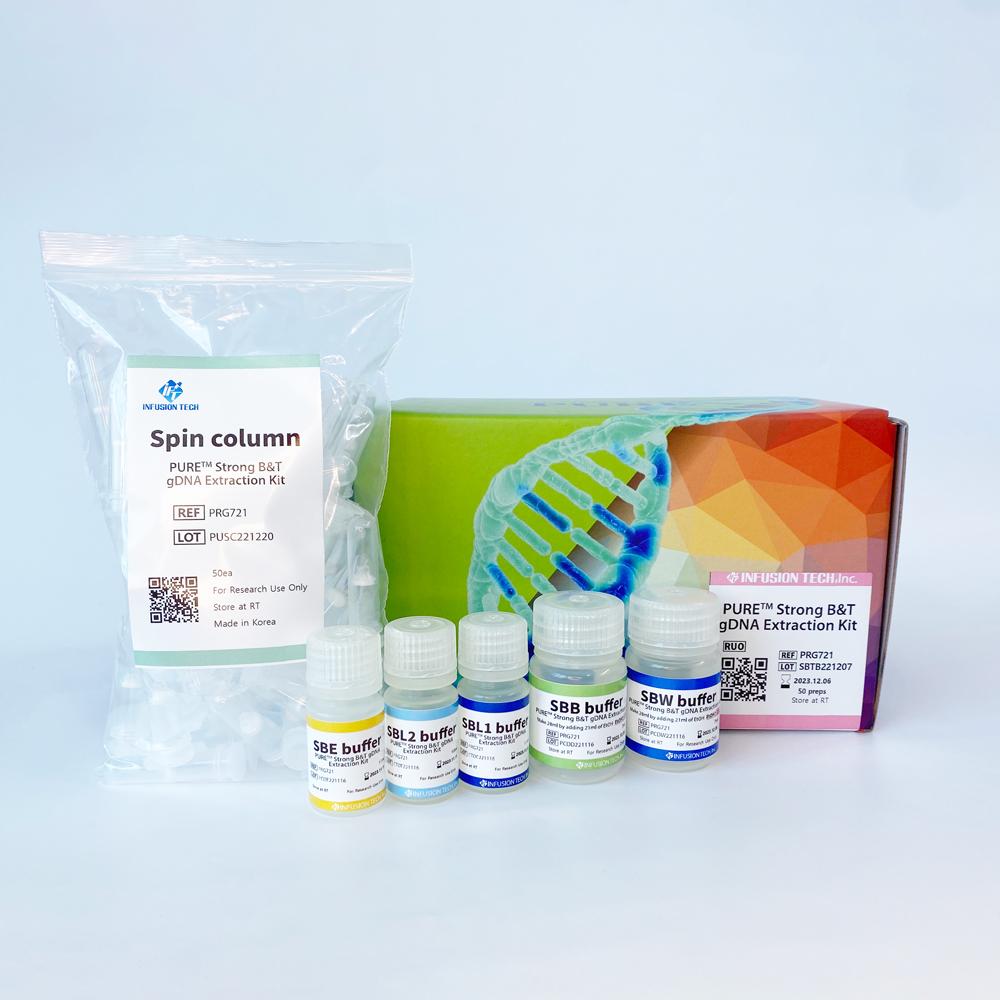
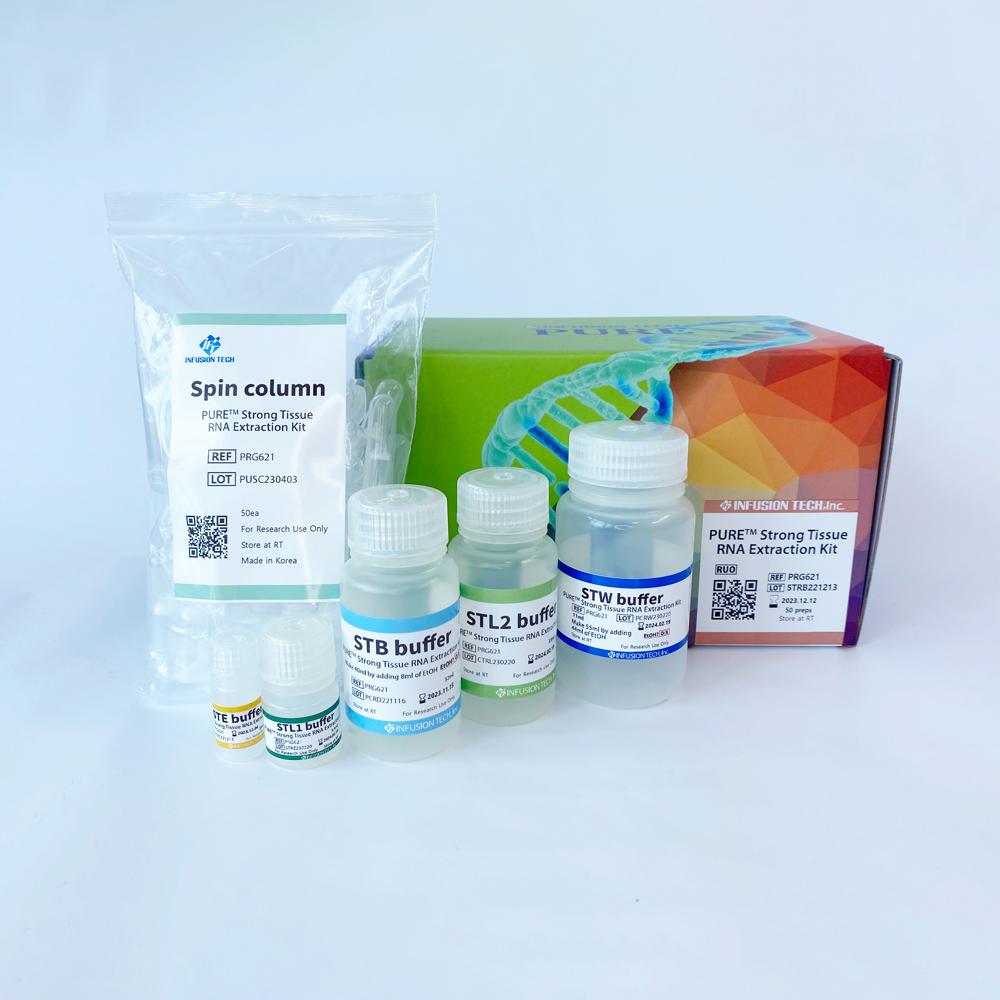
PURE™ Strong Blood&Tissue NA Extraction Kit
-
Payment
OT , T/T , WK
-
MOQ
1 Set
-
Supply Ability
500 Set per One-Time
-
Supply Details
Customization
What we can produce with our current staff and equipment.
-
Country of sale
Americas, Asia, Middle East, Europe, Africa
-
PRICE
-
FOB
USD 433.00
(200 Set)
-
ITEM SPECIFICS
-
Brand
Model PRG721~, PRG621~PURE
-
origin
Republic of Korea
-
Size(Capacity)
50 preps / 100 preps / 200 preps
-
Color
clear
-
Weight
450g / 600g / 1200g
-
Expiry Date
1year
-
Features
Blood&Tissue NA Extraction kit
PRODUCT DESCRIPTION
As lysis time is short, rapid extraction of high-concentraction and high-purity gDNA and RNA are possible.
• Unneeded to use shredder column in the lysis step
• Unnecessary to perform phenol, chloroform or alcohol precipitation
• High-purity gDNA and RNA are extracted(A260/A280 : 1.8~2.0)
| Product Name | Cat.No. | Size |
| PURE™ Strong B&T gDNA Extraction Kit | PRG721 | 50 preps |
| PRG721H | 100 preps | |
| PRG721H2 | 2x100 preps | |
| PURE™ Strong Tissue RNA Extraction Kit | PRG621 | 50 preps |
| PRG621H | 100 preps | |
| PRG621H2 | 2x100 preps |
| Product Name | PURE™ Strong B&T gDNA Extraction Kit |
| Package | 50 tests/kit, 100 tests/kit, 200 tests/kit |
| Storage condition | Can be stored at room temperature up to 1 year. Proteinase K may be stored at room temperature before initial use. After dissolution with distilled water, recommended to aliquot and store at –20°C in order to avoid repeated freezing and thawing. |
| Sample types that can be used | A variety of animal tissues, cultured cells, gram-positive and negative bacteria, and plasma/serum/buffy coat samples can be used. If results are not good, we recommend to use our PURE™ Strong B&T gDNA Extraction kit, which uses polymer-based lysis buffer of great efficiency. |
| MOQ | No MOQ required |
| Certificates of manufacturing facility | ISO9001, ISO14001 |
Participated in the 2022 Chicago Clinical Diagnosis Exhibition Expo [ 2022 AACC ]
Participated in the 2022 Indonesia Export Consultation
Participated in the 2022 German Medical Device Exhibition [ 2022 MEDICA ]
Participated in 2023 Dubai Medical Equipment Exhibition [ 2023 MEDLAB ]
Exhibited in 2023 Anaheim Clinical Diagnosis Exhibition Expo [ 2023 AACC ]
1. What are the features of the PURE™ series that make it different from other extraction products?
A : Other products use pretreatment(shredder) columns and require two types of spin columns, resulting in a long or complicated extraction process. In addition, some products use organic solvents such as phenol and chloroform. The PURE™ series developed by Infusion Tech does not use pretreatment columns and enables high-purity, high-concentration nucleic acid purification with a single spin column. The precipitation process using organic solvents is also not required, making it a quick and easy three-step process (lysis, wash, elute).
2. What type of samples can be used for extraction?
A : Various samples including cultured cell(104~108 cells), tissue(25~50mg), bacteria(<109 cells), blood & body fluids(<200μl) can be used.
3.What should I do if blood samples are very viscous?
A : If the sample is very viscous, please add 1X PBS (not supplied), Proteinase K in an increased volume than the volume specified in the manual.
4. Is it possible to extract gDNA from whole blood samples?
A: This product was optimized to work well using plasma, serum, and buffy coat samples. It could be difficult to extract from whole blood.
5. I need RNA free-genomic DNA. Is there any extraction method for this purpose?
A: If you need RNA free-genomic DNA please add 4μl of RNase A (100mg/ml, not provided) on the same step Proteinase K is added, according to the manual.
6. Is it possible to perform follow-up experiments (NGS, Sequencing, PCR) with the PURE™ Cell&Tissue gDNA Extraction Kit?
A : PURE™ Cell&Tissue gDNA Extraction Kit has the advantage of extracting high purity and high concentration of nucleic acids, so it is possible to perform subsequent experiments. Extracted gDNA can be used in various experiments including NGS, sequencing, PCR, electroporation, and cloning.
7. Are the components of the PURE™ Cell&Tissue gDNA Extraction Kit sterile?
A: The components of the PURE™ Cell&Tissue gDNA Extraction Kit are sterile.
8. Which buffers in the PURE™ Cell&Tissue gDNA Extraction Kit do I add ethanol to?
A: DB buffer and Wash buffer should be used after adding ethanol. If you want to check for other components, please refer to the MSDS of the product.
9. How is product quality control (QC) performed for the PURE™ Cell&Tissue gDNA Extraction Kit?
A: The QC of all products is performed by randomly selecting products of the same LOT after production and performing extraction using our standard sample. The test methods are quantification, electrophoresis, and PCR. Products are released if they meet our criteria.
10. How do I use lyophilized components (Proteinase K)?
A: The lyophilized components should be used after adding the appropriate amount of distilled water specified in the manual and completely dissolving the powder. After initial dissolution and use, store at –20℃. It is recommended to store in aliquots as performance may decrease if they go through repeated freezing and thawing.
11. The filter was clogged while using it, why?
A: The amount of sample used to perform extraction is presumed to be high. We recommend re-experimenting within the sample volume range specified in the manual. If the amount of sample is small, it is likely that a low concentration will be measured when quantitating, so please reduce the amount of elution buffer.
12. There are white powder on the wall of the spin column. Is there something wrong with the product?
A : The powder on the wall of the spin column is filter powder caused by static electricity during the manufacturing process. When the solution pass through the filter, the powder will be caught by the filter, and will not influence purity of the eluted DNA.
Our business is centered on the diagnostic business, which is aimed at rapid and accurate diagnosis by effectively extracting DNA/RNA of the causative pathogen for infectious diseases such as human fungi, rickettsial diseases, and scabies mites that have emerged after the corona pandemic, and the research business, which manufactures nucleic acid extraction reagents for research for government offices, research institutes, and university institutions.
We are connected to various buyers through various medical device exhibitions, export fairs, and consultations to expand our business not only in Korea but also overseas, and we are building an agency to expand our business.
∙ Factory Information
Our manufacturing facility is a KGMP manufacturing facility certified by the Korean Ministry of Food and Drug Safety and is managed by the ISO 9001,14001,13485 quality management system.
∙ Our Service
Manufacturing, distribution, and trading of PURE (extraction reagents for research), PINA (extraction reagents for IVD), and Primera (molecular diagnostic reagents for IVD).
R&D CERTIFICATE
PAYMENTS DETAILS
- WK
- OT
- Telegraphic Transfer : T/T
- Name : NAMHEON KIM
SHIPPING
- 38 Heungan-daero 427beon-gil Dongan-gu, Anyang-si, Gyeonggi-do (14059)
The person in charge
NAMHEON KIMAddress
38 Heungan-daero 427beon-gil Dongan-gu, Anyang-si, Gyeonggi-do (14059)
⠀⠀
-
- Business Type :
- Manufacturer
-
- Main Product :
- PINATM POC NA Extraction kit
-
- Established :
- 2020-03-11
-
- Total Annual Revenue :
- 2~3 billion (KRW)
-
- Total Employees :
- 11~50 people
R&D CERTIFICATE
COMPANY ENVIRONMENT
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Please enter the text on the left image to prevent automatic input.
0 / 4000
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- 2~3 billion (KRW)
-
- Total export revenue (previous year in USD)
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
- MEMBER
- INFUSION TECH, Inc. Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★