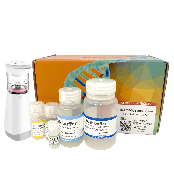
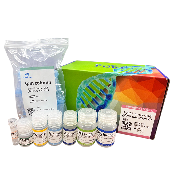
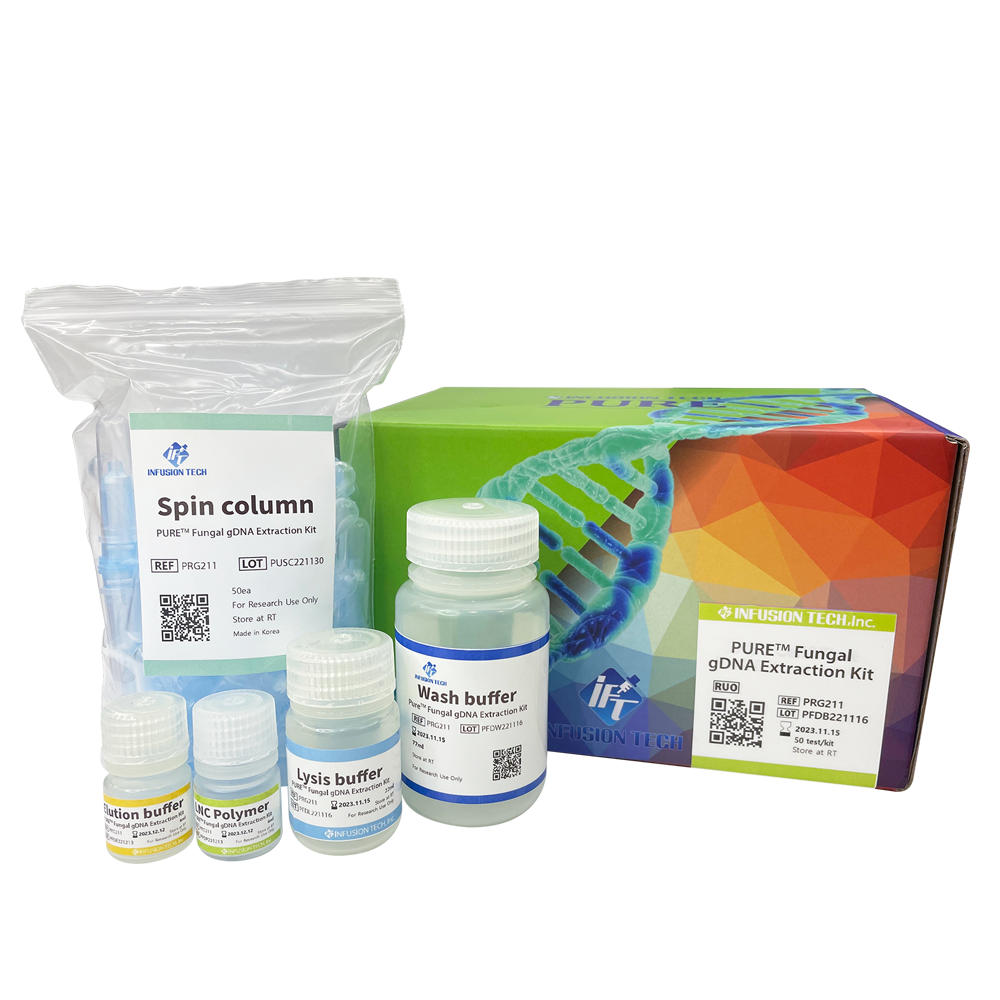
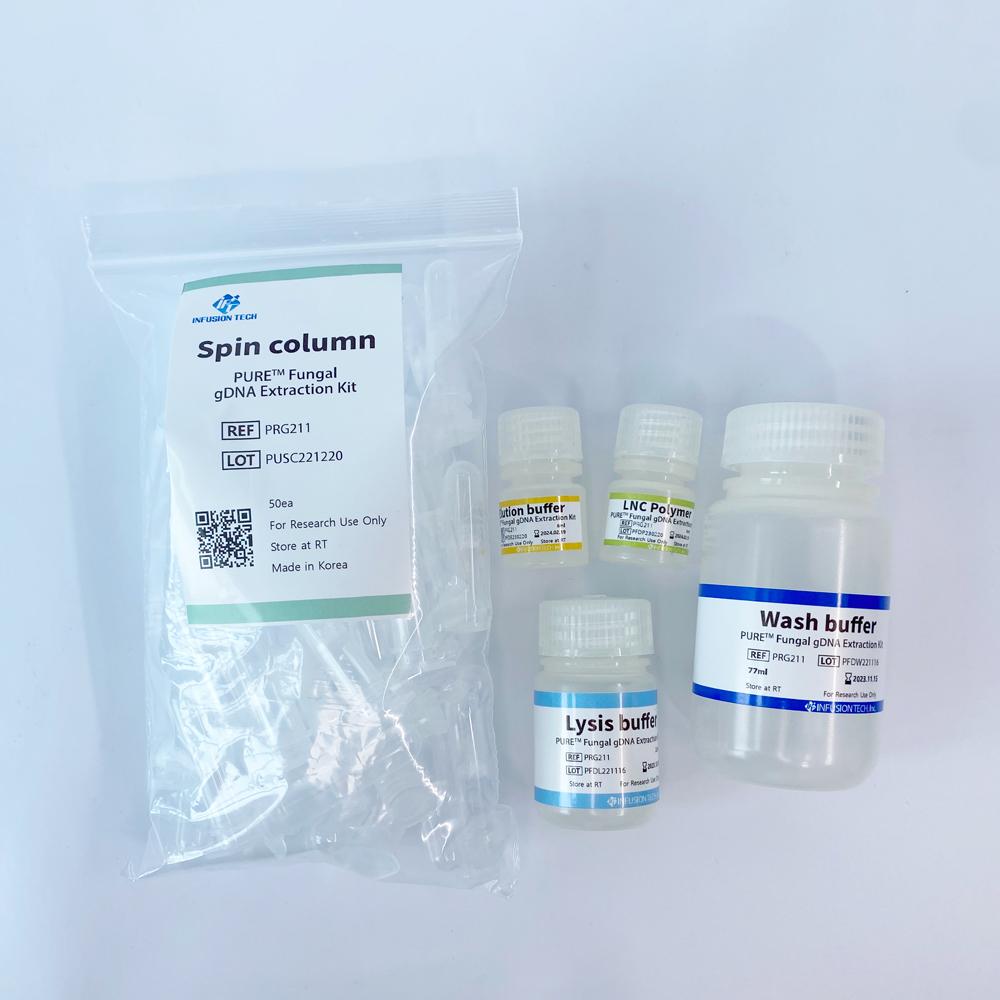
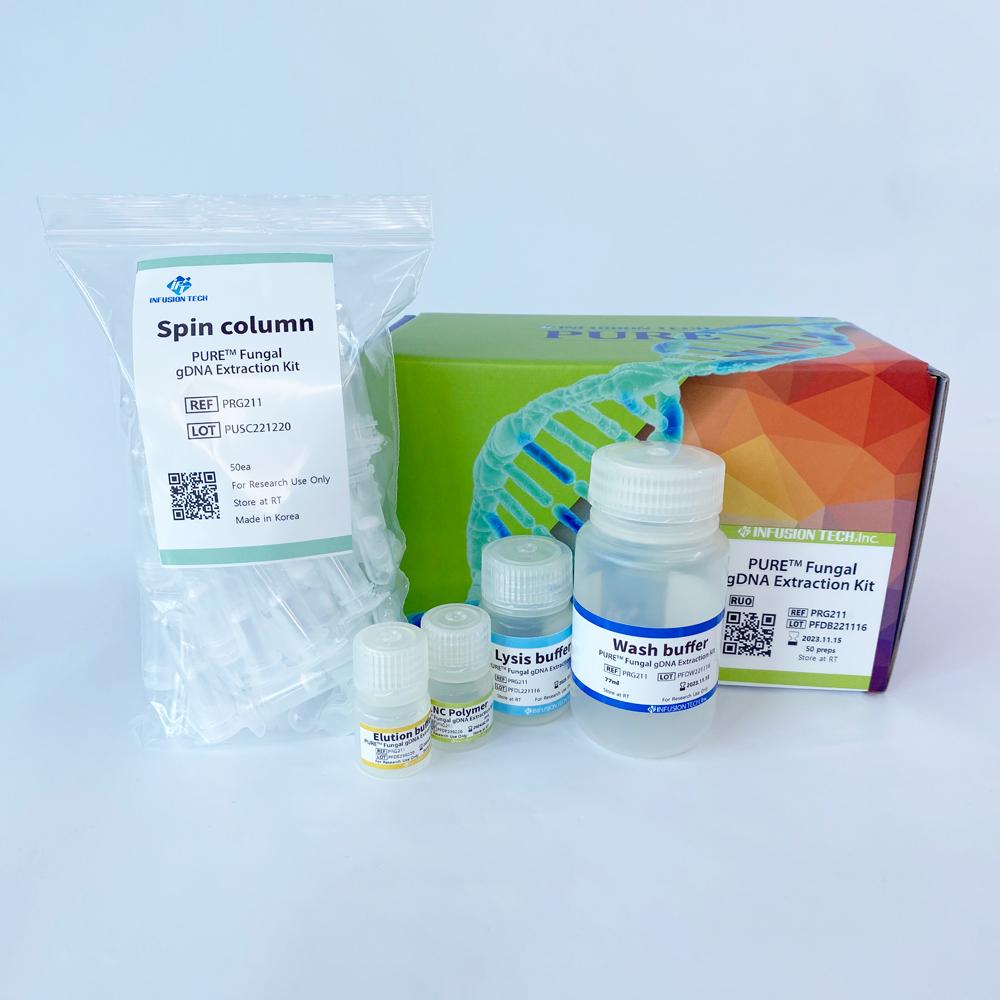
PURE™ Fungal gDNA Extraction Kit
-
Payment
T/T
-
MOQ
50 Set
-
Supply Ability
10,000 Set per One-Time
-
Supply Details
Customization Sample Order
50prep/1kit, 100prep/1kit, 200prep/1kit
-
Country of sale
Americas, Asia, Middle East, Europe, Africa
-
PRICE
-
FOB
USD 575.00
(20 Set)
-
ITEM SPECIFICS
-
Brand
Model PRG211~PURE
-
origin
Republic of Korea
-
Size(Capacity)
50prep/20.5*12*12, 100prep/20.5*12*12, 200prep/20.5*12*12 x 2 Box
-
Material
plastic bottle + reagent + spin column + packaging box
-
Color
clear
-
Weight
450g / 600g / 1,200g
-
Expiry Date
1 year
-
Features
NA Extraction kit
PRODUCT DESCRIPTION
PURE™ Fungal gDNA Extraction Kit
Optimized for simple and effective gDNA extraction from fungi species. Extracted gDNA can be used for subsequent experiments including PCR, qPCR and sequencing.
Product Features
• Lysis time reduced : lysis and capture of gDNA on single step
• Lysis at room temperature possible due to patented polyemr
• Safe use : organic solvents are not used
• High-friendliness enhanced : only single type of spin column used
• Lysis at room temperature possible due to patented polyemr
• Safe use : organic solvents are not used
• High-friendliness enhanced : only single type of spin column used
Product Specification
| Product Name | Cat.No. | Size |
| PURE™ Fungal gDNA Extraction Kit | PRG721 | 50 preps |
| PRG721H | 100 preps | |
| PRG721H2 | 2x100 preps |
| Package | 100 tests/kit |
| Storage condition | Can be stored at room temperature up to 1 year. |
| Sample types that can be used | Cultured fungi, plasma, serum |
| MOQ | No MOQ required |
| Certificates of manufacturing facility | ISO9001, ISO14001, ISO13485 |
Product history (relevant product export history and exhibition participation history)
Participated in the 2021 German Medical Device Exhibition [ 2021 MEDICA ]
Participated in the 2022 Chicago Clinical Diagnosis Exhibition Expo [ 2022 AACC ]
Participated in the 2022 Indonesia Export Consultation
Participated in the 2022 German Medical Device Exhibition [ 2022 MEDICA ]
Participated in 2023 Dubai Medical Equipment Exhibition [ 2023 MEDLAB ]
Exhibited in 2023 Anaheim Clinical Diagnosis Exhibition Expo [ 2023 AACC ]
Participated in the 2022 Chicago Clinical Diagnosis Exhibition Expo [ 2022 AACC ]
Participated in the 2022 Indonesia Export Consultation
Participated in the 2022 German Medical Device Exhibition [ 2022 MEDICA ]
Participated in 2023 Dubai Medical Equipment Exhibition [ 2023 MEDLAB ]
Exhibited in 2023 Anaheim Clinical Diagnosis Exhibition Expo [ 2023 AACC ]
Samples validated with this kit
Cultured fungus(maximum 100mg), fungus spiked to blood : Penicillium corylophilum, Penicillium commune, Rhizopus arrhizus(oryzae), Rhizopus stolonifer, Mucor racemosus, Candida albicans, Candida auris, Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, Aspergillus terreus, Aspergillus oryzae, Aspergillus tubingensis etc.
Fungi culture of various species, for product validation
Lysis procedure compared with existing methods
Patented polymers, how do they perform lysis?
LNC(Lysis and capture) polymers chemically bind to fungal cell walls through charge difference, physically disrupts cell walls when vortexing is performed and biologically induces cell death. At the same time extracted nucleic acids are captured by these polymers. Due to their excellent performance heating is not required on lysis steps.
A: Synthesized polymer
B: Untreated Aspergillus fumigatus spore cells
C: Polymer adsorbed to A.fumigatus spore cells causing disruption
QC data
DNA was successfully extracted even at low concentrations, when Aspergillus terreus was serially diluted (106 cells/ml~103 cells/ml)
Company Overview
We are an in vitro diagnostic medical device company that specializes in manufacturing nucleic acid pretreatment reagents.
Our business is centered on the diagnostic business, which is aimed at rapid and accurate diagnosis by effectively extracting DNA/RNA of the causative pathogen for infectious diseases such as human fungi, rickettsial diseases, and scabies mites that have emerged after the corona pandemic, and the research business, which manufactures nucleic acid extraction reagents for research for government offices, research institutes, and university institutions.
We are connected to various buyers through various medical device exhibitions, export fairs, and consultations to expand our business not only in Korea but also overseas, and we are building an agency to expand our business.
∙ Factory Information
Our manufacturing facility is a KGMP manufacturing facility certified by the Korean Ministry of Food and Drug Safety and is managed by the ISO 9001,14001,13485 quality management system.
∙ Our Service
Manufacturing, distribution, and trading of PURE (extraction reagents for research), PINA (extraction reagents for IVD), and Primera (molecular diagnostic reagents for IVD).
Our business is centered on the diagnostic business, which is aimed at rapid and accurate diagnosis by effectively extracting DNA/RNA of the causative pathogen for infectious diseases such as human fungi, rickettsial diseases, and scabies mites that have emerged after the corona pandemic, and the research business, which manufactures nucleic acid extraction reagents for research for government offices, research institutes, and university institutions.
We are connected to various buyers through various medical device exhibitions, export fairs, and consultations to expand our business not only in Korea but also overseas, and we are building an agency to expand our business.
∙ Factory Information
Our manufacturing facility is a KGMP manufacturing facility certified by the Korean Ministry of Food and Drug Safety and is managed by the ISO 9001,14001,13485 quality management system.
∙ Our Service
Manufacturing, distribution, and trading of PURE (extraction reagents for research), PINA (extraction reagents for IVD), and Primera (molecular diagnostic reagents for IVD).
Other Products
R&D CERTIFICATE
PAYMENTS DETAILS
This supplier supports payments for offline orders
- Telegraphic Transfer : T/T
- Name : NAMHEON KIM
SHIPPING
Shipping from :
Republic of Korea
- 38 Heungan-daero 427beon-gil Dongan-gu, Anyang-si, Gyeonggi-do (14059)
INFUSION TECH, Inc.
The person in charge
NAMHEON KIMAddress
38 Heungan-daero 427beon-gil Dongan-gu, Anyang-si, Gyeonggi-do (14059)
⠀⠀
INFUSION TECH, Inc.
Introduction
INFUSION TECH, Inc. is a nucleic acid extraction company that has its own patented extraction technology that is different from conventional methods of nucleic acid extraction.
The main business areas consist of research, diagnostic, and industrial products.
Research : PURE Series
Diagnostic : PINA Series, Primera Series
Industrial : PURE, PERI Series
Our products enable the purification of highly concentrated gDNA and RNA through the nucleic acid capture method using patented materials, ensuring a high yield that allows the use of several times more samples, and enabling nucleic acid extraction in the shortest time with a quick and easy experimental method.
In addition, to realize rapid and accurate molecular diagnosis, the product has been thoroughly verified and researched for effective nucleic acid extraction according to each type of sample by infectious disease, and is manufactured and quality controlled within the KGMP, ISO13485, ISO9001, ISO14001 quality management system to maintain consistent quality.
We are connected to various buyers through various medical device exhibitions, export fairs, and consultations to expand our business not only in Korea but also overseas, and we are building an agency to expand our business.
∙ Factory Information
Our manufacturing facility is a KGMP manufacturing facility certified by the Korean Ministry of Food and Drug Safety and is managed by the ISO 9001,14001,13485 quality management system.
∙ Our Service
Manufacturing, distribution, and trading of PURE (extraction reagents for research), PINA (extraction reagents for IVD), and Primera (molecular diagnostic reagents for IVD).
-
- Business Type :
- Manufacturer
-
- Main Product :
- PINATM POC NA Extraction kit
-
- Established :
- 2020-03-11
-
- Total Annual Revenue :
- 2~3 billion (KRW)
-
- Total Employees :
- 11~50 people
R&D CERTIFICATE
COMPANY ENVIRONMENT
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- 2~3 billion (KRW)
-
- Total export revenue (previous year in USD)
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
- MEMBER
- INFUSION TECH, Inc. Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★